T-cell Development and Normal Subsets:
Early in T-cell development, T-lymphocytes rearrange the T-cell receptor (TCR) genes, leading to expression of the heterodimer TCRαβ or TCRγδ.
TCRαβ represent ~95% of all T-cells and include 2 major subsets T-helper and T-cytotoxic cells.
- Generated from CD4+CD8+ thymocytes undergoing positive selection through interaction of the TCR with self-peptide MHC class I/II only thymocytes with low-avidity TCRs survive and leave the thymus.
- There are 4 major CD4 and CD8 T-cell maturation stages:
- Naïve
- Central memory
- Effector memory
- Effector cells
Normal T-cell subsets
- Follicular T-helper cells (Tfh)
- CD4+ memory T-cells
- Predominantly in germinal centres where they interact with B-cells
- At least three from: BCL6, PD-1, CXCL13, CD10, ICOS/CD278, CCD5/CD195
- Neoplastic counterpart: angioimmunoblastic T-cell lymphoma and other nodal lymphomas of Tfh origin
- Regulatory T-cells (Treg)
- Immunological tolerance against self and foreign antigens
- Usually 1-2% of circulating CD4+ T-cells
- Expression of CD25high, CD127low, FOXP3
- Neoplastic counterpart: Adult T-cell leukemia/lymphoma (ATLL)
- TCRγδ
- Represent ~5% of lymphocytes
- Predominantly in the spleen, liver and mucosal tissues; role in innate and adaptive immune response
- Increased in autoimmune diseases, inflammatory disorders, infections, other hematological malignancies
- Immunophenotype:
- CD3+, CD4-, CD8-/dim+
- Express all T-cell antigens (CD2, CD3, CD5, CD7)
- Often CD57+, less often CD16+, CD56+; subsets can express CD94
- Reactive changes include complete or partial loss of CD5, dim CD7 and bright CD3 expression.
- Overlapping features with TCRγδ-derived T-cell lymphomas
CD4:CD8 ratio
- Peripheral blood: typically 1-3 for adults, normal range 0.8-6 (Porwit and Béné, 2018)
- Bone marrow: can be lower than in PB, but usually also between 1-3
- Lymph node and tonsils: higher than in PB, with predominance of CD4+ T-cells in reactive states
- Spleen: similar to PB
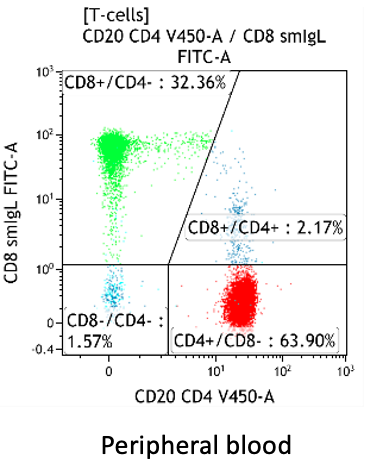
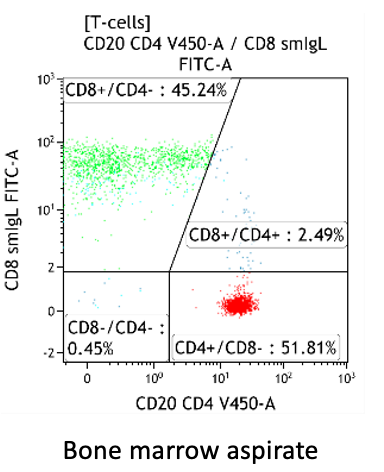
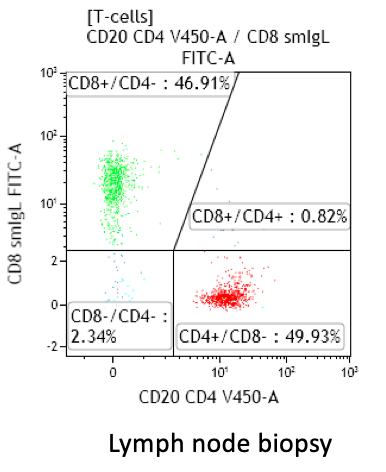
Increased CD4:CD8 ratio
- Infectious/inflammatory conditions, autoimmune disorders, medication effect
- Consider malignant processes with a reactive T-cell component (e.g. Hodgkin lymphoma with increased CD4+ T-cells)
Decreased CD4:CD8 ratio
- Viral infection (typical in CMV, EBV), autoimmune disease, post-transplantation
- T-cell large granular lymphocytic leukemia; consider if persistent increase in CD57+ T-cells, B-symptoms, cytopenias, organomegaly
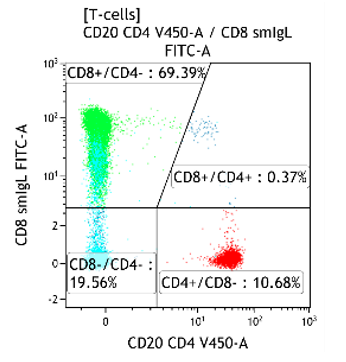
Fig 1. CMV reactivation with a CD4:CD8 ratio of 0.15 and increased CD4-/CD8- T-cells
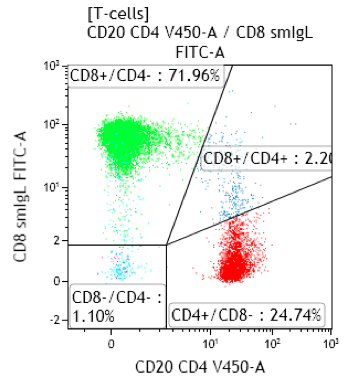
Fig 2. Infectious mononucleosis with a CD4:CD8 ratio of 0.34
Normal/reactive variations:
- Small CD5- and CD7- T-cell subsets are frequent
- Late memory T-cells typically lose CD7
- Increased CD7- subsets in chronic viral infections, inflammatory skin disorders, autoimmune diseases and in the elderly
- Dim CD5 is a feature of CD8+ and TCRγδ subsets
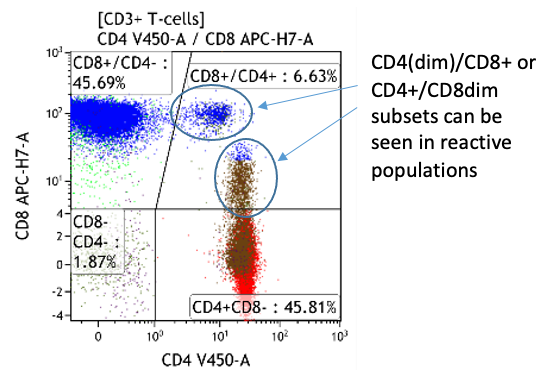
- CD4+CD8+ T-cells normally <1% of peripheral blood T-cells
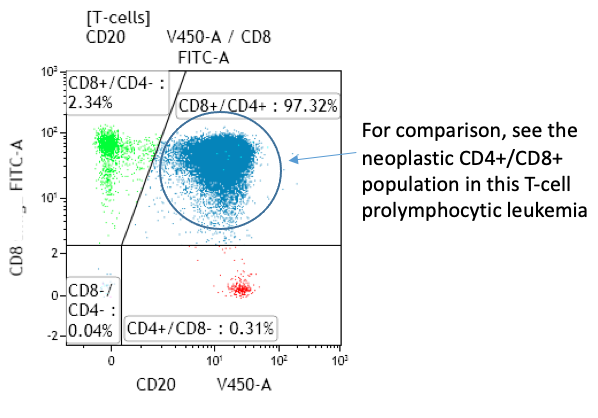
- Expansion of CD4bright+/CD8weak+ T-cells sometimes seen in neoplastic disorders
- Expansion of CD4weak+/CD8bright+ typical of infectious mononucleosis
Infectious mononucleosis
- Typical immunophenotype in acute infection: massive expansion of CD2high, CD7low, CD38high, HLA-DRhigh, CD28+/-low, CD45ROhigh, CD45RA-/+low, CD11b-/+low, CD11c+/-low, negative for cytotoxic markers
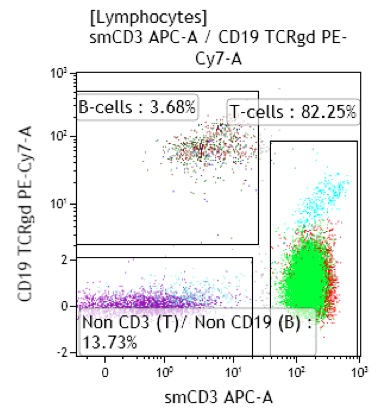
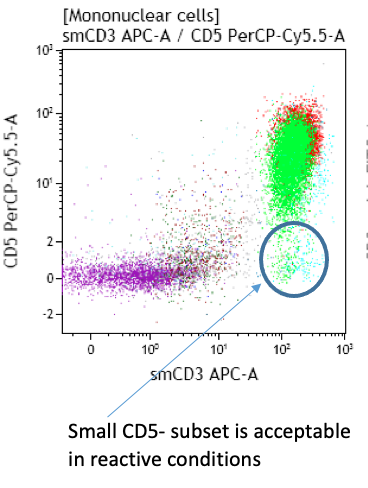
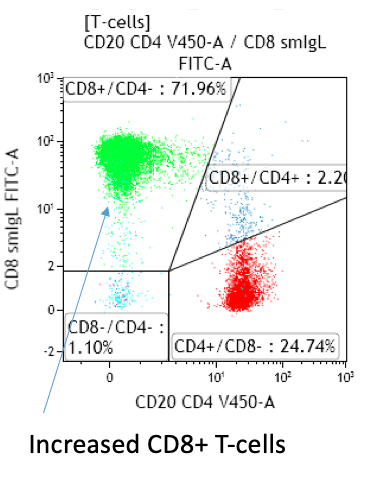
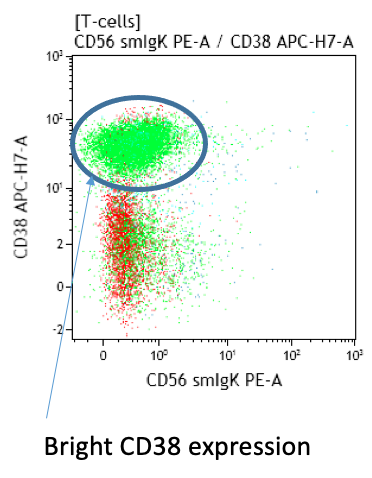
Peripheral blood; lymphocytosis with predominance of T-cells:
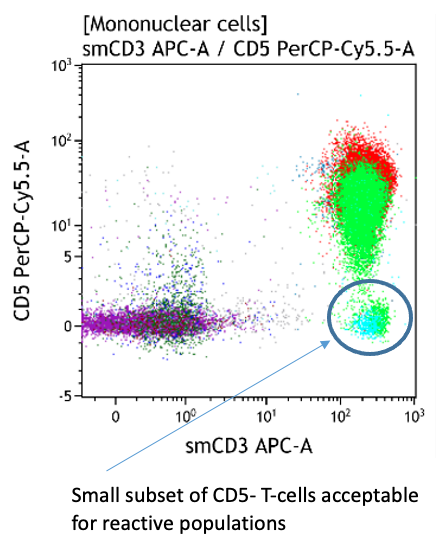
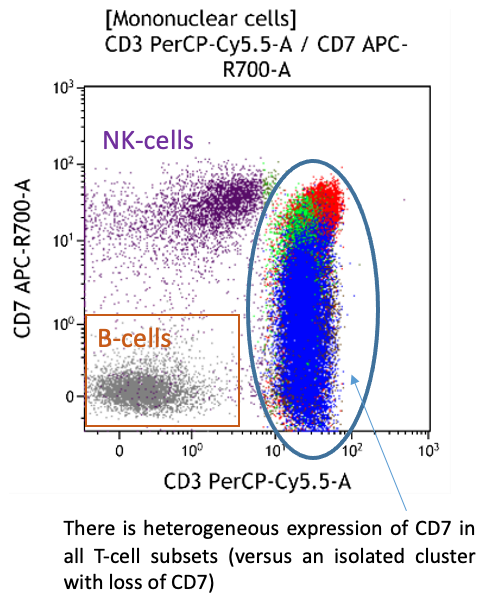
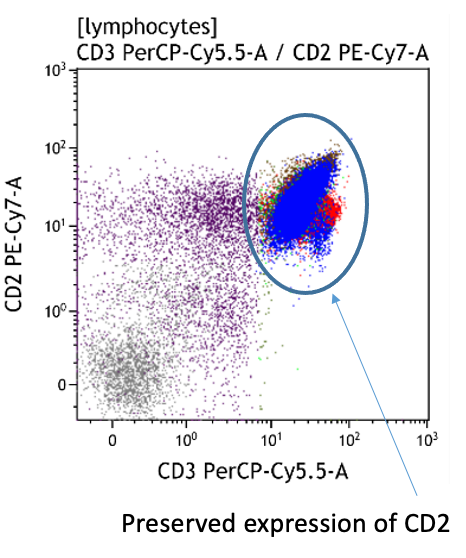
Both CD4+CD57+ and CD8+CD57+ subsets are present.
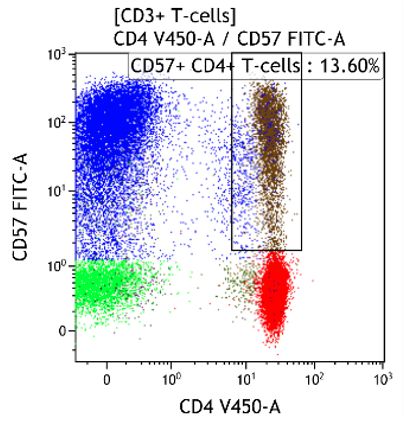
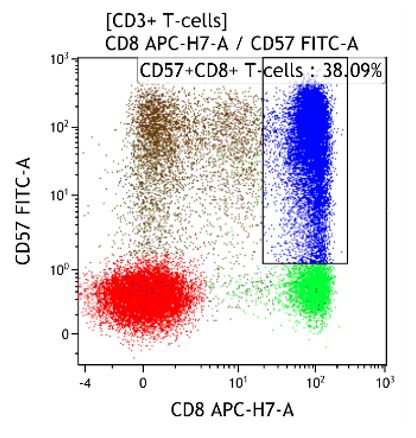
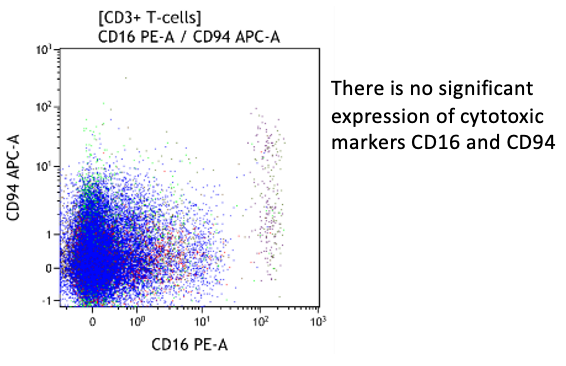
Heterogeneous T-cell populations, no definitive immunophenotypic aberrancies; favor reactive, clinical correlation required, recommend follow-up in 6 months to monitor the evolution. If clinical concern molecular studies for T-cell clonality can be considered.
Next page: Natural killer cells (NK-cells)
